16 October 2020
An evaluation of new procedures for getting parents’ or young people’s consent to the human papillomavirus (HPV) vaccination has found some evidence of improved uptake of the vaccine but results were mixed. The evaluation, led by researchers at the NIHR Health Protection Research Unit in Behavioural Science and Evaluation at University of Bristol, concluded that increasing uptake in low-uptake populations remains a challenge and there are unresolved issues in relation to self-consent.
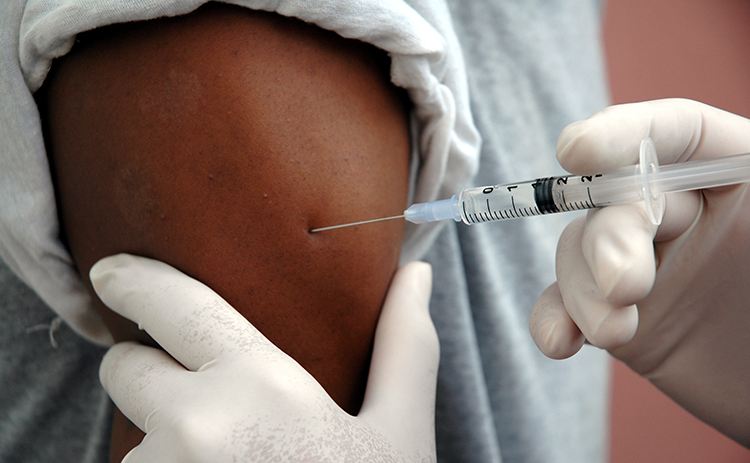
The English schools-based HPV vaccination programme aims to prevent death and illness from HPV-related diseases, including cervical cancer. Since introduction in 2008, high coverage has been achieved amongst vaccine-eligible young women, with over 10 million receiving immunisation against HPV. However, there are pockets of lower uptake among some populations – such as minority ethnic groups and those educated in alternative provider settings.
The requirement for written parental consent has been shown to be a barrier to vaccination. In England, the legal framework allows young people to be vaccinated without parental consent provided they are deemed ‘Gillick-competent’, that is, have the maturity to make their own decisions and to understand the implications of those decisions. However, an earlier study shows adolescent self-consent was not widely implemented.
New consent procedures were introduced in 2017–2018 in two local authorities in the south–west of England because of concerns about low uptake rates. The new procedures started with a request for written parental consent but also included contacting parents to ask for verbal consent, and the opportunity for adolescent self-consent if parents could not be contacted.
The study team at the University of Bristol, Sirona Care and Health, Public Health England and University Hospitals Bristol NHS Foundation Trust, undertook a mixed methods study to explore the impact on uptake and acceptability of the new consent procedures. The research was led by Drs Suzanne Audrey & Harriet Fisher from the NIHR Health Protection Research Unit in Behavioural Science and Evaluation at University of Bristol.
Summary of quantitative findings
We carried out statistical analyses to examine changes in uptake of the HPV vaccination programme.
We compared HPV vaccination uptake in intervention sites, implementing the new procedures, with other sites in England. We found that uptake in one of the local authorities increased from 76.3% to 82.5% (2017/18-2018/19). Further analysis showed the effect was 11.5% when compared with local authorities with similar characteristics, but not implementing the intervention, because on average their uptake decreased over the same period (the difference-in-difference effect). But we did not find evidence for change in uptake in the other local authority.
We also carried out an analysis to compare uptake of the HPV vaccination programme within the intervention local authorities by ethnicity, type of school and level of deprivation. However, we did not find any evidence for a difference ‘before’ and ‘after’ the consent procedures were implemented.
Dr Harriet Fisher, Senior Research Associate at the University of Bristol, said: ‘We found some evidence that implementing the new consent procedures can improve uptake, and overcome trends for decreasing uptake, of the HPV vaccine. However, we still need to find a way to address inequalities in uptake which is of concern because of the disproportionate effect of HPV-related cancers among minority ethnic and more deprived groups.’
Summary of qualitative findings
We also undertook interviews with 53 participants to ask for their view about the new consent procedures. This included four healthcare professionals involved in the delivery of the HPV vaccination programme, eight school staff, 19 young women aged 12 to 17 years and 22 parents.
We found that the preferred age at which the HPV vaccination is offered, 12 to 13 years, contributed to a reluctance in endorsing self-consent. It was difficult for respondents to decide whether this age is the right time to self-consent because of perceived differences in the maturity of Year 8 students. One parent said: “Some 13-year olds can make really sensible decisions, and others you can’t trust them to go to the shop.”
The potential for new consent procedures to highlight the right to refuse vaccination was also a concern. One young women said: “I don’t think we’re allowed to make the decision completely by ourselves, and I don’t think we should either because most kids will go ‘Oh, it’s a needle, I’ll go out of it, I won’t do it’ but it’s actually quite important.”
There were also concerns about the impact of self-consent on relationships within families and between professionals and the families they serve. One school staff member at an alternative education provider said: “I suppose ultimately parental relationships are really important to us… I would hate to drive a wedge in between us and the family.”
Dr Suzanne Audrey, Senior Research Fellow in Public Health in Bristol Medical School, said: “This study has highlighted unresolved issues in relation to adolescent self-consent for the HPV vaccination and potentially other vaccines and healthcare initiatives for young people. These include public and professional perceptions of young people’s rights and abilities to take responsibility for decisions affecting their health.”
The study was funded by National Institute for Health Research (NIHR) Research for Patient Benefit Programme.
Papers:
Impact of new consent procedures on uptake of the schools-based human papillomavirus (HPV) vaccination programme by Harriet Fisher et al. Published in Journal of Public Health. September 2020
How acceptable is adolescent self-consent for the HPV vaccination: Findings from a qualitative study in south-west England by Suzanne Audrey et al. Published in Vaccine. October 2020.
Further information
About the NIHR
The National Institute for Health Research (NIHR) is the nation’s largest funder of health and care research. The NIHR:
- funds, supports and delivers high quality research that benefits the NHS, public health and social care
- engages and involves patients, carers and the public in order to improve the reach, quality and impact of research
- attracts, trains and supports the best researchers to tackle the complex health and care challenges of the future
- invests in world-class infrastructure and a skilled delivery workforce to translate discoveries into improved treatments and services
- partners with other public funders, charities and industry to maximise the value of research to patients and the economy.
The NIHR was established in 2006 to improve the health and wealth of the nation through research, and is funded by the Department of Health and Social Care. In addition to its national role, the NIHR supports applied health research for the direct and primary benefit of people in low- and middle-income countries, using UK aid from the UK government.